Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESOURCE): a randomized, double-blind, placebo-controlled, phase 3 trial
Summary:
Hepatocellular carcinoma (HCC) locoregional treatment includes surgical resection, transplantation, ablation and chemoembolization. For those patients who are not candidates for such treatment, medical management with the oral multikinase inhibitor sorafenib, can provide improvement in overall survival. This study aimed to evaluate the safety and efficacy of regorafenib, for patients in whom HCC has progressed during treatment with sorafenib.
Across 152 centers and 21 countries, 573 patients with progression of HCC on sorafenib were enrolled in a randomized 2:1 regorafenib to placebo, double-blind, placebo-controlled phase 3 clinical trial. The primary endpoint was overall survival analyzed by intention to treat. Median overall survival was 10.6 months (95% CI 9.1 – 12.1) with regorafenib and 7.8 months (6.3 – 8.8) with placebo. Median progression-free survival was 3.1 months (2.8 – 4.2) with regorafenib and 1.5 months (1.4 – 1.6) with placebo by mRECIST criteria; median time to progression of 3.2 months (2.9 – 4.2) and 1.5 months (1.4 – 1.6) respectively. The safety profile of regorafenib in HCC is consistent with the use of this medication in other malignancies. The most common reason for discontinuation of regorafenib was disease progression (60%, 83% in the control group).
This study concludes that regorafenib provides a clinical improvement in overall survival in patients with progression of HCC while on sorafenib. Two patients had a complete tumor response by mRECIST criteria. This suggests that the sequential use of two multikinase inhibitors with partially overlapping target profiles can provide benefit to this patient population.
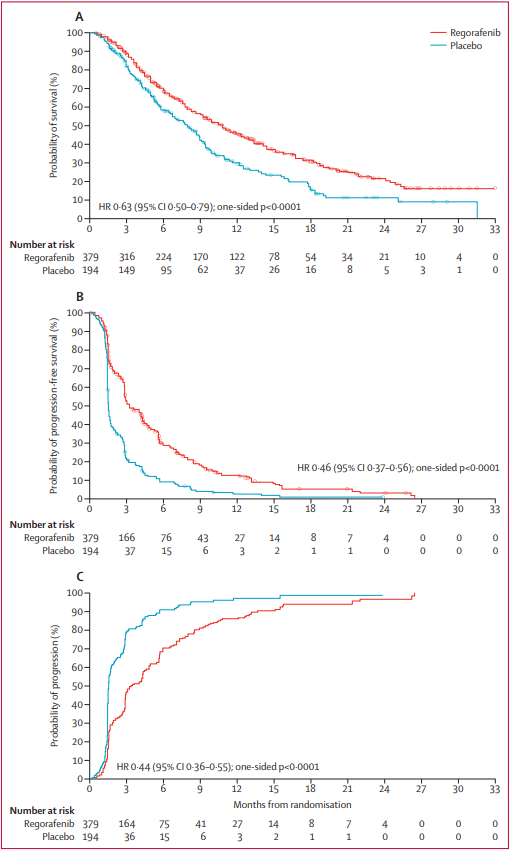
Figure 2. Kaplan-Meier analysis of overall survival (A), progression-free survival (mRECIST; B), and time to progression (mRECIST; C)
Commentary:
Regorafenib offers a viable option for patients who have failed sorafenib and has the potential to add to the current treatment armamentarium. Multiple clinical trials with second line therapies including TACE (Kudo et al., GIDEON trial, Zhang et al.), HAIC (SIRIUS trial, Terashima et al., Shao et al.) and alternative medications (Llovet et al., Zhu et al.) have demonstrated variable benefit depending on the treatment timing or drug.
The STAB Study Phase II data, recently published by Sato et al. indicates a similar class of patients (Child-Pugh class A with Barcelona Stage C disease) may benefit from concurrent TACE with sorafenib therapy with a mean overall and progression free survival of 17.3 and 5.4 months respectively, longer than those with regorafenib therapy. While this patient population was chemotherapy-naïve, it offers a potential treatment option for those patients on sorafenib prior to treatment failure. The introduction of regorafenib has the potential to change IR treatment for refractory HCC as concurrent regorafenib-TACE or alternative IR therapies may become a viable option in the future.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESOURCE): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 389: 56-66.
Post Author:
Nicole A. Keefe, MD
Resident Physician
Department of Radiology and Medical Imaging
University of Virginia
@NikkiKeefe
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.